The Future of Medicine
Starts Here.
Optimum Clinical Research Group is now enrolling participants for phase I-IV clinical trials at our newly opened, state-of-the-art facility in Albuquerque, NM.
OUR FACILITY
Purpose Built to Move Medicine Forward
We are dedicated to conducting clinical research in a better, more efficient way. We have accomplished this by integrating research capacity into our building, clinic, community, and administrative infrastructure. As a community-based private facility, we have leveraged our community relationships with our local radiology, lab, specialist & sub-specialists, life science, and hospital partners to provide the same service and capacity you would find at an academic medical center. We are biotherapy approved by the NIH and are gene, biologic, and viral therapy ready with Advarra’s IBC.
When we built our newly designed state-of-the-art facility, we built with research in mind. Our clinic boasts an on-site class B1 compounding pharmacy, large infusion suite, research dedicated exam rooms, research dedicated lab that is integrated into the clinic area, and dedicated monitoring space. We have made investments in adopting a 21 CFR part 11 compliant eSource, eReg, CTMS platform called Clinical Research IO. This means all data is captured in real-time at the computer or on a tablet. Regulatory binders and logs are electronically custom built to match the traditional paper binder. Studies are custom made according to the Sponsor’s eCRF guidelines with data alert and limit features to seamlessly facilitate site-level compliance, cleaner data, and more compliant and efficient end-to-end trial conduct so therapies can get to patients faster.
ABOUT US
Driven by Innovation, Guided by Compassion
Our goal is to provide our Sponsor and research partners with the clinical research capacity of an Academic Medical Center with the speed and efficiency of a privately owned practice. Our Investigators have more than 15 years of clinical research experience on phase I-IV complex therapeutic, investigator-initiated, academic, cooperative group, and industry-sponsored trials. While our partner clinic has historically always focused on solid tumors and gynecologic malignancies, we are actively working on expanding our research and trial offerings to include women’s health & gynecology, primary care, exercise medicine, hyperbaric therapy, nutrition, and regenerative and integrative therapeutics.
Our clinic has participated in 60 + industry-sponsored, PI-initiated, academic, and NCI-NCTN clinical trials; many of these therapies have gone on to receive FDA approval, and patients are now benefiting from these treatments. Our research team leverages more than 50 combined years of experience on more than 150 trials across almost all therapeutic areas from primary care, rheumatology, immunology, psychiatry, neurology, neurosurgery, women’s health, oncology (all indications), surgical trials, device trials, trauma, burns, acute care, transplant, translational & pre-clinical studies, cancer control and prevention, public health, and health services trials.
We have built a robust clinical research infrastructure. We are committed to providing our study participants with a safe, enjoyable research experience in a deeply caring environment while also providing our research and industry partners with clean, useable data.
Together with the help of our partners and sponsors, we are moving medicine forward because patients can’t wait.

WHAT MAKES US DIFFERENT
Primed to Take Clinical Research Forward
Our facility is purpose-built to integrate research into clinical care, we are primed to move medicine forward:
An on-site gynecologic oncology and women’s health clinic
On-site sterile compounding pharmacy

Real-time data capture ability with the use of eSource & eReg through Clinical Research IO (CRIO)
100% remote monitoring capability and dedicated monitoring space for on-site visits
Extensive storage capacity in our pharmacy & laboratory including -80 to -20 freezers, refrigerated, and ambient storage

Access to a large, diverse patient population and referral network ranging from El Paso to Colorado and Arizona
A large infusion suite

Biotherapy approved by the NIH and Gene Therapy Ready with Advarra IBC
On-Site Hyperbaric suite, integrative therapies clinic, and human performance center
Research dedicated exam rooms
Research dedicated lab with refrigerated & ambient centrifuge, microcentrifuge, incubator, calibrated pipettes, wet bench space, dry ice, liquid nitrogen, and tissue banking ability

Deeply experienced and extensively credentialed clinical researchers, investigators, investigational pharmacist, nursing, research administrative, and clinic staff
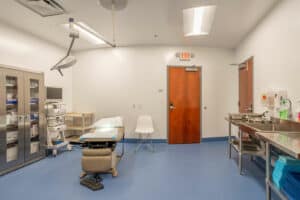
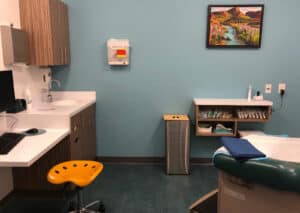
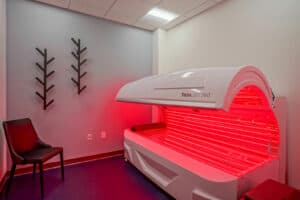
GALLERY
Take a Tour of our State-of-the-Art Facility























GET INVOLVED
Sponsor Inquiries
Your sponsorship can change lives. Let’s talk.
